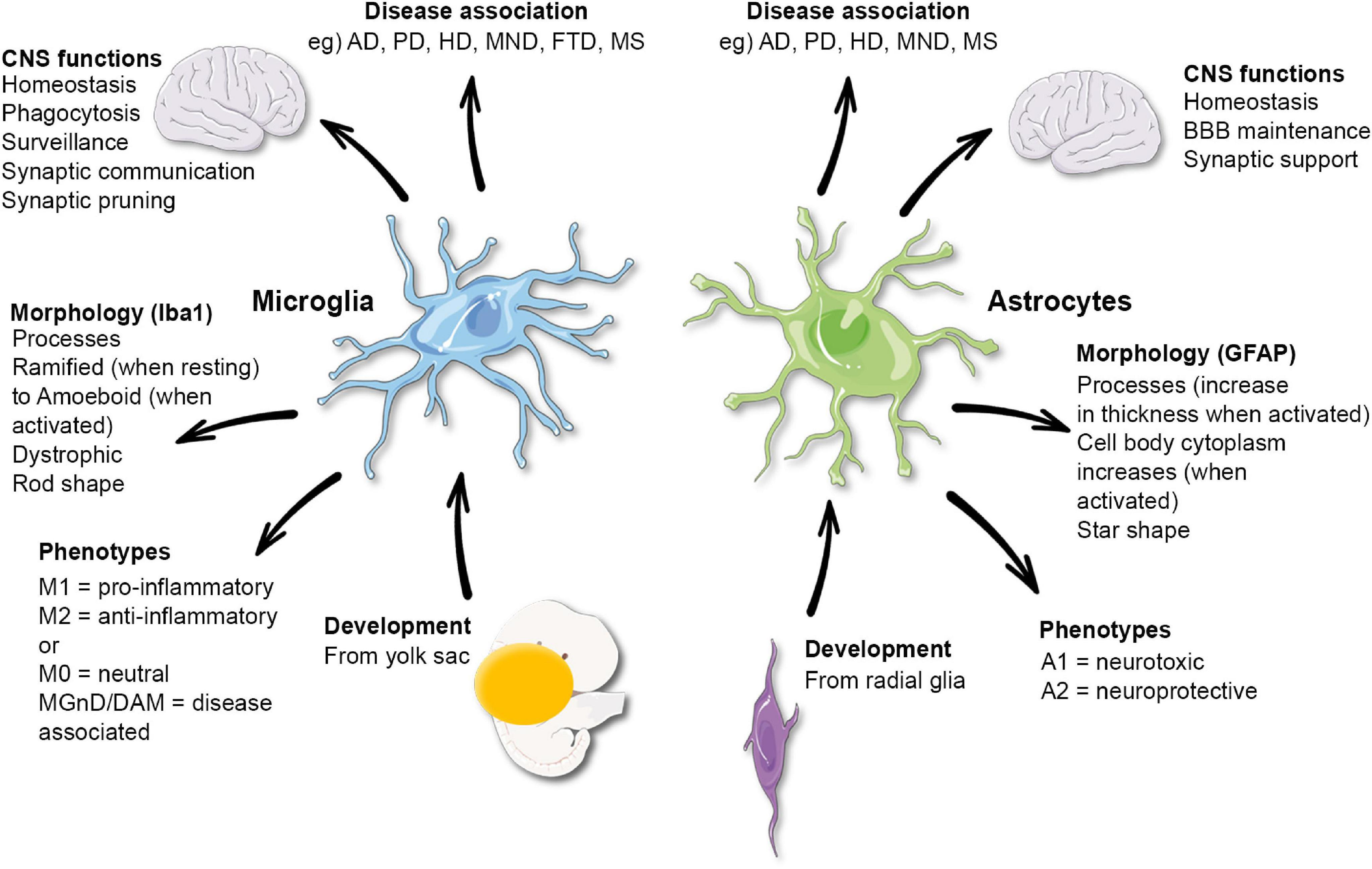
Microglial Research: Transforming Alzheimer’s Disease Care
admin May 5, 2025Science News ArticleMicroglial research is at the forefront of understanding the brain’s immune system, especially in the context of neurodegenerative diseases like Alzheimer’s disease. Led by prominent neuroscientist Beth Stevens, this field has revealed how microglial cells play a vital role in maintaining brain health by identifying and clearing damaged or dead cells. However, when these immune cells malfunction, they can inadvertently contribute to the progression of disorders such as Alzheimer’s and Huntington’s disease. The groundbreaking findings from the Stevens Lab have not only shed light on the mechanisms behind abnormal synaptic pruning but also paved the way for potential new biomarkers and therapeutic strategies. As over 7 million Americans grapple with Alzheimer’s disease, ongoing microglial research holds promise for developing innovative treatments that could change the landscape of neurology research.
The exploration of microglial cells—the brain’s resident immune warriors—offers exciting insights into how these cells influence neurobiology and contribute to the onset of various brain disorders. The investigations spearheaded by leading researchers like Beth Stevens delve into the intricate functionalities of these cells, revealing their dual role in maintaining neural circuitry while also posing potential threats to neuronal health when their activity becomes dysregulated. This line of inquiry is pivotal to our understanding of conditions such as Alzheimer’s disease, which continues to challenge the medical community. By focusing on the cellular dynamics within the brain’s immune framework, scientists are uncovering vital links between microglial dysfunction and neurodegenerative diseases. Such knowledge not only advances the field of neurology but also ignites hope for effective interventions that could mitigate the impact of these debilitating conditions.
Understanding Microglial Research in Alzheimer’s Disease
Microglial cells are integral to maintaining the health of the brain, functioning as its immune system. In Alzheimer’s disease, these cells can become dysfunctional, leading to harmful processes such as aberrant synaptic pruning. This phenomenon is crucial in understanding how certain neurodegenerative diseases progress, and the pioneering work of researchers like Beth Stevens is shedding light on these mechanisms. By exploring the dual nature of microglial activity, where they protect and also potentially harm brain health, Stevens is carving a new pathway in neurology research that not only advances scientific knowledge but also opens avenues for therapeutic interventions.
Stevens’s research emphasizes that while microglia act as caretakers by clearing out dead cells and providing essential nutrients to neurons, their improper activation can exacerbate conditions like Alzheimer’s disease. Her lab’s findings have highlighted the importance of identifying biomarkers linked to microglial dysfunction, which serves as a critical step toward developing targeted treatments. The interconnectedness of microglial research and other neurodegenerative diseases showcases the necessity for a comprehensive approach in neuroscience that could potentially lead to breakthroughs in how we understand and tackle these conditions.
The Role of the Brain Immune System in Neurodegenerative Diseases
The brain’s immune system, largely comprised of microglial cells, plays a vital role in combating neurodegenerative diseases such as Alzheimer’s. As the first line of defense, these cells constantly monitor for signs of distress or damage within the brain. Their primary functions include the removal of cellular debris and the regulation of synaptic connectivity, which are critical processes for maintaining cognitive functions. However, in conditions like Alzheimer’s, microglial cells can ironically contribute to disease progression due to their improper activation resulting in excessive inflammatory responses.
Understanding the mechanisms behind microglial function offers insights into the pathology of Alzheimer’s disease. The work done by Stevens and her team underscores the significance of this brain immune system, revealing how dysfunction can accelerate cognitive decline. As we continue to unravel the complexities of neuroinflammation, the hope is to develop innovative treatment strategies that can mitigate the detrimental effects of microglial dysregulation and ultimately improve the quality of life for millions affected by neurodegenerative disorders.
Insights from Beth Stevens on Neurology Research and Alzheimer’s
Beth Stevens’ journey as a neurology researcher is remarkable, demonstrating the potential of curiosity-driven science to unravel the complexities of the human brain. Her focus on microglial cells has led to groundbreaking findings that link immune response to neurodegenerative diseases. Her early work, often considered foundational, highlights how basic research—such as studying the visual systems in mice—can yield profound insights into human brain disorders. This exploration has challenged the traditional boundaries of neurological research, showing that what may seem irrelevant today could pave the way for critical treatments tomorrow.
By advocating for continued funding and support for basic science, Stevens emphasizes the interconnected nature of research and its impact on clinical outcomes. The engagement and investment in this area are pivotal for advancing our understanding of diseases like Alzheimer’s. Through her work, she aims not only to unravel the scientific questions surrounding microglial dysfunction but also to inspire future generations of scientists to pursue innovative avenues in their explorations of the brain.
The Impact of Federal Funding on Neurology Research
Federal funding plays a crucial role in advancing neurology research, particularly in the exploration of conditions like Alzheimer’s disease. As articulated by Beth Stevens, the financial support from institutions such as the National Institutes of Health has been instrumental in her research trajectory. This backing not only facilitates the exploration of complex questions but also encourages scientists to venture into uncharted territories of knowledge that could ultimately lead to groundbreaking discoveries. The story of Stevens’s lab exemplifies how stable funding can support long-term, curiosity-driven research initiatives.
Moreover, the investment in fundamental research is essential for translating scientific discovery into clinical application. Without adequate resources, vital studies into microglial function and their roles in neurodegenerative diseases would struggle to get off the ground. Therefore, advocating for and maintaining robust federal funding is paramount—it ensures that researchers can probe deeper into the mechanisms of diseases like Alzheimer’s and mobilize their findings into potential therapeutic advancements that could benefit those living with these debilitating conditions.
Transformative Discoveries in Neurodegenerative Disease Landscapes
The field of neurodegenerative disease research is undergoing a transformation largely driven by discoveries about microglial cells. As highlighted by Beth Stevens, these cells are not merely passive bystanders in brain pathology but active participants in synaptic pruning and immune response. This realization has shifted the scientific community’s perspective, prompting a reevaluation of how such immune functions impact diseases like Alzheimer’s and Huntington’s. By recognizing the active role of microglia, researchers can develop more targeted therapeutic strategies that specifically address their dysregulation.
As scientists delve deeper into the interactions between microglial activity and neurodegenerative processes, the potential for innovative treatment modalities increases. This could lead to the identification of novel biomarkers that enable earlier diagnosis or the development of drugs that precisely modulate microglial functions. Stevens’ work exemplifies the ripple effect that one transformative discovery can have, highlighting the need for interdisciplinary approaches that amalgamate insights from immunology, neurology, and genetics to tackle the complexities of brain health comprehensively.
Challenges and Opportunities in Alzheimer’s Research
The journey to understanding Alzheimer’s disease is fraught with challenges, yet fertile with opportunities for breakthroughs. One major hurdle is the complexity of the brain’s immune system, specifically regarding how microglia interact with various neuronal pathways. As Stevens points out, unraveling these relationships requires a blend of patience, creativity, and substantial groundwork laid by earlier research. Acknowledging the difficulties is essential; however, it is equally important to recognize the vast potential for new insights that such investigations can yield.
Moreover, as researchers like Stevens emphasize, collaborative efforts across disciplines can enhance our understanding of Alzheimer’s disease significantly. New technologies, interdisciplinary approaches, and continued funding can augment the research landscape, allowing scientists to make connections that were previously overlooked. By staying committed to addressing these challenges with innovative solutions, we pave the way towards effective treatments that could alter the course of Alzheimer’s disease and improve lives.
Linking Synaptic Pruning to Neurodegenerative Disease Progress
A significant area of exploration in neurology research involves the link between synaptic pruning and the progression of neurodegenerative diseases. Beth Stevens’s work illustrates how microglial cells, while essential for maintaining neural health, can induce synaptic pruning that may lead to neuronal loss if left unchecked. This understanding underscores the delicate balance required for healthy brain function and highlights how disruptions in normal microglial activity could potentially lead to conditions such as Alzheimer’s.
The correlation between excessive synaptic pruning and neurodegenerative pathology opens up new avenues for therapeutic intervention. By targeting the mechanisms of microglial action, researchers may develop strategies that can prevent improper pruning, thereby preserving synaptic integrity and cognitive function. Stevens’s focus on this balance encourages a deeper inquiry into the underlying processes governing microglial activity, promising to advance our capabilities in managing neurodegenerative diseases more effectively.
The Future of Therapeutic Interventions in Alzheimer’s Disease
As the landscape of Alzheimer’s disease research evolves, so too does the development of therapeutic interventions aimed at ameliorating symptoms and slowing disease progression. With insights garnered from microglial research, scientists are better positioned to create targeted therapies that address the root cause of neurodegenerative conditions. This could revolutionize treatment, moving away from symptomatic care to addressing the pathological mechanisms at play.
Beth Stevens’s groundbreaking findings are paving the way for innovative treatment paradigms, potentially leading to personalized medicine approaches tailored to the individual’s unique neurobiological profile. Future therapies might include modulators that enhance normal microglial function, offering hope to patients navigating the challenges of Alzheimer’s disease. By fostering a culture of collaboration and innovation in the field, we can embrace the future of Alzheimer’s research with optimism and ambition.
The Critical Interplay Between Basic and Translational Research
The synergy between basic and translational research is vital in the quest to understand and treat neurodegenerative diseases. Researchers like Beth Stevens highlight the importance of foundational scientific investigations that explore the fundamental biology of microglia and their roles in brain health. Basic research not only unpacks intricate cellular mechanisms but also lays the groundwork for developing practical applications that can be translated into clinical settings.
This interplay is essential in areas such as Alzheimer’s research, where the gap between laboratory findings and patient care can be significant. Stevens’s work illustrates that, through rigorous exploration of neuronal and immunological interactions, scientists can identify viable pathways for intervention. The continual flow of insights from basic research into clinical practice is crucial for revolutionizing our approach to complex diseases like Alzheimer’s, ultimately enhancing patient outcomes and innovative treatment options.
Frequently Asked Questions
What role do microglial cells play in Alzheimer’s disease research?
Microglial cells are crucial to Alzheimer’s disease research as they function as the brain’s immune system, patrolling for damage and clearing dead cells. Research led by Beth Stevens has shown that improper pruning by microglia can contribute to neurodegenerative diseases like Alzheimer’s and Huntington’s disease, paving the way for new treatment strategies.
Who is Beth Stevens and what is her contribution to microglial research?
Beth Stevens is a prominent neuroscientist and investigator focused on microglial research related to Alzheimer’s disease. Her innovative work has transformed our understanding of how microglial cells affect synaptic pruning and are involved in neurodegenerative disorders, potentially leading to new biomarkers and therapeutic approaches.
How do microglia affect the brain’s immune system in neurodegenerative disease?
Microglia are integral to the brain’s immune system, helping to maintain homeostasis by removing dead neurons and regulating synaptic connections. In neurodegenerative diseases like Alzheimer’s, dysregulation of microglial activity can lead to harmful synaptic pruning, aggravating disease progression.
What is the significance of basic science in microglial research according to Beth Stevens?
Beth Stevens emphasizes that basic science is essential for advancing microglial research, as it fosters curiosity-driven studies that explore fundamental questions. These investigations, often supported by NIH funding, are critical for uncovering the biological mechanisms of neurodegenerative diseases and developing effective treatments.
What are the potential implications of microglial research for treating Alzheimer’s disease?
Research on microglia has significant implications for treating Alzheimer’s disease. By understanding how aberrant microglial activity affects neuronal health and contributes to disease pathology, scientists aim to develop targeted therapies that can mitigate damage, improve outcomes, and enhance the quality of life for those affected.
How has microglial research evolved in recent years?
In recent years, microglial research has evolved significantly, shifting from a mere understanding of their role in brain health to recognizing their involvement in neurodegenerative diseases like Alzheimer’s. This evolution has been driven by groundbreaking studies led by researchers like Beth Stevens, which have highlighted the potential of microglia as therapeutic targets.
What are some challenges facing microglial research in neurodegenerative diseases?
Microglial research faces challenges such as understanding the complexity of microglial functions in the brain, the need for better models to study their behavior in the context of neurodegenerative diseases, and translating findings into effective treatments for diseases like Alzheimer’s, which currently lack a cure.
What future directions can we expect from microglial research?
Future directions in microglial research may involve identifying specific pathways that microglia use in neurodegenerative diseases, developing new biomarkers for early detection of conditions like Alzheimer’s, and discovering novel therapeutic strategies that harness or modulate microglial activity to enhance brain health and function.
| Key Points | Details |
|---|---|
| Importance of Microglial Cells | Microglia act as the brain’s immune system, monitoring for illness and helping to clear damaged cells. |
| Aberrant Pruning | Dysfunctional pruning by microglia is linked to neurodegenerative diseases like Alzheimer’s and Huntington’s. |
| Research Support | Stevens’ research has been largely funded by NIH, highlighting the importance of governmental support in scientific advancements. |
| Public Health Impact | The research aims to impact care for 7 million Americans affected by Alzheimer’s disease, which currently has no cure. |
| Basic Science Role | Initial research trajectories without apparent outcomes can lead to significant discoveries and new treatment possibilities. |
Summary
Microglial research is pivotal in understanding brain health and its associated disorders. Beth Stevens’ groundbreaking studies demonstrate that microglial cells, the immune guardians of the brain, are vital in maintaining neural integrity. She emphasizes that the exploration of basic science underpins significant advances in treatment options for Alzheimer’s and related diseases. The ongoing investigations in microglial behavior hold the promise for new biomarkers and therapies, showcasing how foundational research in this area is essential to improving outcomes for millions living with neurodegenerative conditions.
You may also like
Archives
Calendar
| M | T | W | T | F | S | S |
|---|---|---|---|---|---|---|
| 1 | ||||||
| 2 | 3 | 4 | 5 | 6 | 7 | 8 |
| 9 | 10 | 11 | 12 | 13 | 14 | 15 |
| 16 | 17 | 18 | 19 | 20 | 21 | 22 |
| 23 | 24 | 25 | 26 | 27 | 28 | 29 |
| 30 | 31 | |||||