Bile imbalances and liver cancer are crucial topics in modern medicine, particularly as researchers unravel the complex mechanisms linking bile acid metabolism to hepatocellular carcinoma (HCC). Recent studies highlight how dysregulation in bile production can contribute to serious liver diseases, including the most common form of liver cancer. A critical discovery involves the role of the FXR, or Farnesoid X receptor, which plays a fundamental part in maintaining bile acid homeostasis. When YAP signaling pathway is activated inappropriately, it can inhibit FXR’s ability to function properly, leading to an overproduction of bile acids that exacerbate liver inflammation and ultimately promote cancer development. As the understanding of these pathways evolves, it opens new doors for innovative liver cancer treatment options, highlighting the need for further investigation into bile acids and their influence on liver health.
The exploration of bile acid disorders and their correlation with hepatic malignancies is gaining momentum in the scientific community. Biliary dysfunctions, characterized by abnormal bile compositions, have been implicated in the onset of various liver diseases, including hepatocellular carcinoma (HCC). Insights into the FXR’s regulatory functions shine a light on how imbalances in bile production can disrupt metabolic homeostasis and increase cancer risk. Additionally, the interplay between the Hippo/YAP signaling pathway and bile acid regulation underscores the complex biological landscape that researchers are navigating. By delving into these intricate relationships, we may uncover novel therapeutic strategies for treating liver cancer and improving patient outcomes.
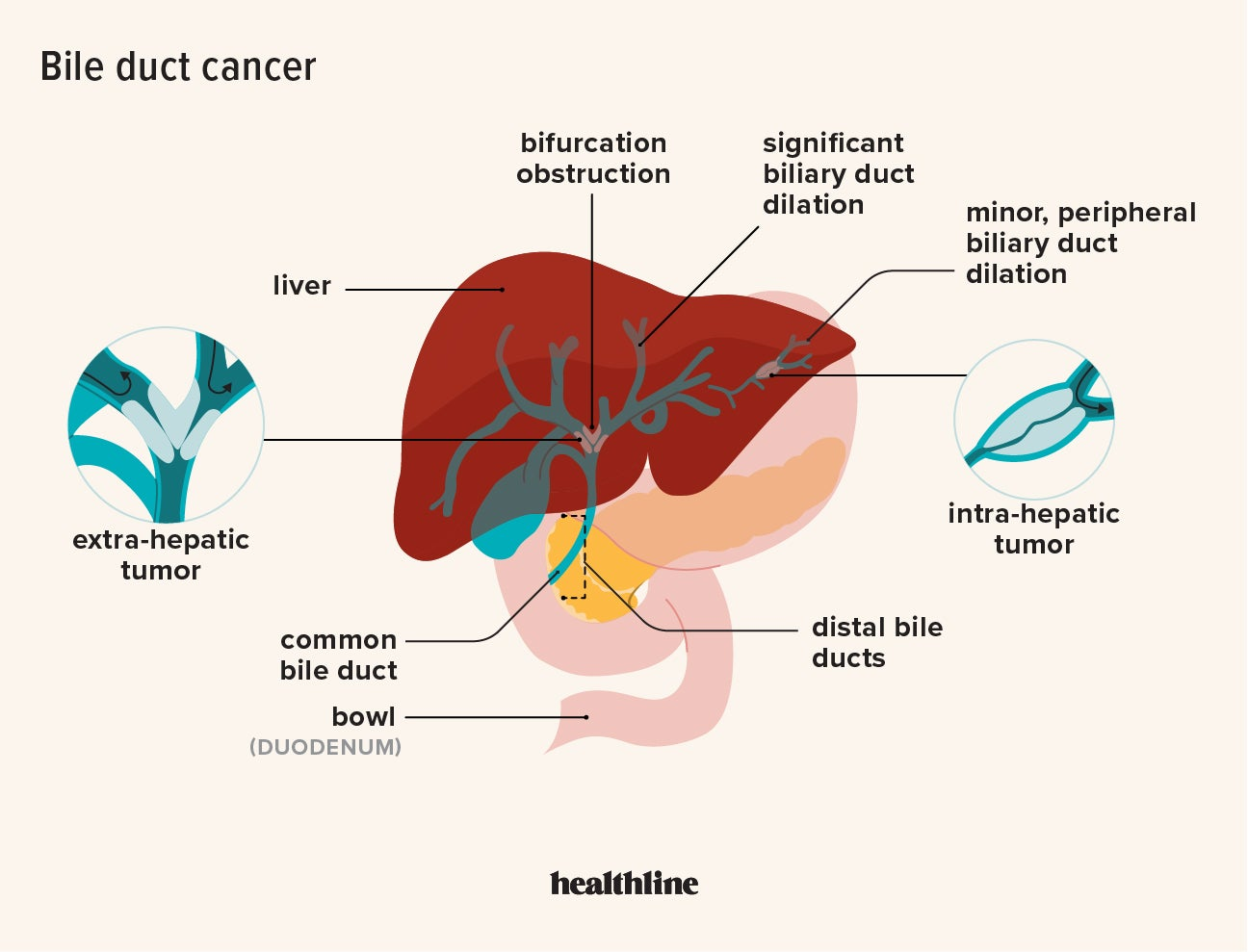
Understanding Bile Acid Imbalances and Their Link to Liver Cancer
Bile acids, integral to fat digestion and nutrient absorption, play a pivotal role in maintaining liver health. An imbalance in these acids can lead to dire consequences, notably liver diseases such as hepatocellular carcinoma (HCC), the predominant form of liver cancer. The liver’s ability to produce and regulate bile acids is critical; when this balance is disrupted, bile acid overload can cause inflammation, fibrosis, and eventually tumor formation, highlighting the necessity of understanding bile acid metabolism in the context of liver cancer.
Research underscores that disrupting the regulatory mechanisms of bile acid production does not merely affect digestion but has profound implications for liver pathology. The recent identification of a key molecular switch revealed by Yingzi Yang’s research illuminates how disturbances in bile acid homeostasis are intimately linked to the development of liver tumors. By understanding how these imbalances occur and their implications, we can begin to explore targeted liver cancer treatment strategies that could effectively address the root causes rather than just the symptoms.
The Role of YAP Signaling Pathway in Liver Cancer Progression
The YAP signaling pathway, primarily known for its role in regulating cell growth and proliferation, has unveiled new complexities in the context of liver cancer. In the research led by Yingzi Yang, it was found that YAP paradoxically acts as a repressor in bile acid metabolism, significantly influencing liver disease outcomes. By inhibiting the functionality of the FXR (Farnesoid X receptor), a vital bile acid sensor, YAP contributes to the overproduction of bile acids — a pathway that leads to liver fibrosis and HCC. Understanding this relationship is crucial for developing novel therapeutic interventions.
Moreover, the implications of YAP signaling extend beyond mere tumor formation; they encompass a broader metabolic framework within the liver. The interaction between YAP and FXR not only shapes the environment conducive to cancer but also alters how the liver handles nutrients, posing challenges in metabolic homeostasis. Targeted therapeutic strategies that can modulate YAP activity, thereby enhancing FXR function or promoting bile acid excretion, hold promise as future liver cancer treatment avenues that could halt disease progression.
Pharmacological Approaches to Modulate Bile Acid and FXR Activity
Addressing the consequences of bile acid imbalances through pharmacological intervention presents exciting possibilities for liver cancer treatment. The study highlighted the potential of activating FXR, an approach that can restore bile acid homeostasis and mitigate liver damage. Enhancing the function of this nuclear receptor not only normalizes bile acid levels but also could reduce the likelihood of tumor formation, offering a multi-faceted strategy for managing hepatocellular carcinoma.
Additionally, the research discusses strategies such as inhibiting HDAC1 or increasing bile acid export proteins (like BSEP), which can quell the deleterious effects of YAP’s repressive actions on bile metabolism. These pharmacological solutions represent a paradigm shift in how we may treat liver diseases linked to bile acid imbalances. By leveraging the understanding of YAP and FXR interactions, new treatments could emerge that specifically target the underlying metabolic disruptions associated with liver cancer.
Impact of Liver Disease Research on Cancer Outcomes
The ongoing research into liver health and diseases underscores the vital link between metabolic processes and cancer outcomes. As scientists reveal more about how bile acids and their regulatory proteins influence liver pathology, it paves the way for innovative cancer therapies. The connection between bile acid dysregulation and cancers such as HCC illustrates that understanding liver diseases holistically is essential for improving patient outcomes.
Further investigation into signaling pathways, like those involving YAP and FXR, will provide deeper insights into how liver diseases evolve into malignancies. The implications of these findings can significantly influence future therapeutic interventions and screening strategies for liver cancer, ultimately aiming for early detection and prevention. By merging knowledge of liver biology with cancer treatment, researchers can foster a more effective approach to combating liver cancer.
The Role of FXR in Liver Health and Disease
Farnesoid X receptor (FXR) is recognized for its pivotal role in maintaining bile acid homeostasis and overall liver health. As a nuclear receptor, FXR regulates the expression of genes involved in bile acid synthesis, transport, and metabolism, thus playing a critical role in preventing lithogenicity and liver injury. Disruption of FXR function has significant implications for liver diseases; it may lead to a cascade of metabolic imbalances and contribute to the pathogenesis of hepatocellular carcinoma.
Recent findings suggest that enhancing FXR activity could serve as a potent strategy for counteracting liver injury and reducing fibrosis. Investigating FXR’s multifaceted role in bile metabolism and potential in regulating YAP signaling opens new avenues for translational research. By developing FXR-targeted therapies, we can re-establish the balance of bile acids, potentially reversing the course of liver disease and improving treatment outcomes for patients affected by liver cancer.
Bile Acid Metabolism and Its Hormonal Functions
Bile acids function not only as emulsifiers in fat digestion but also have hormone-like effects that regulate various metabolic pathways within the body. This dual role highlights the complexity of bile acid metabolism, where disturbances can lead to significant health challenges, including the development of liver cancer. Research demonstrates that when bile acid signaling pathways are disrupted, it can create an environment ripe for tumorigenesis, particularly in hepatocytes.
Understanding the hormonal functions of bile acids, including their interactions with receptors like FXR, emphasizes the importance of bile metabolism in maintaining liver health. The relationship between bile acids and metabolic disorders points toward potential therapeutic approaches that could restore normal metabolic function and prevent the onset of liver cancers. Efforts to harness bile acids’ signaling capabilities may yield novel interventions that target both the digestive and hormonal aspects of liver health.
Exploring Novel Treatments Targeting Bile Acid Overproduction
In light of the critical role bile acids play in liver disease, exploring novel treatments that target bile acid overproduction is paramount. The increasing understanding of molecular mechanisms, particularly the interactions between YAP and FXR, allows for the identification of potential pharmacological agents that can rectify these imbalances. This therapeutic focus could represent a breakthrough in liver cancer treatment, addressing the problem at its root rather than its symptoms.
Experimental models have shown promising results with strategies aimed at reducing bile acid accumulation, such as YAP inhibition or FXR activation. Such approaches not only aim to halt the progression of liver injury but also to prevent the development of cancers such as HCC. Ultimately, finding effective treatments that can modulate bile acid levels will enhance patient prognosis and quality of life for those suffering from liver conditions.
The Future of Liver Cancer Research
The future of liver cancer research looks promising, particularly with ongoing investigations into bile acid metabolism and signaling pathways such as YAP and FXR. As scientists continue to unveil the complexities of liver biology, new potential targets for therapeutic intervention will emerge. The integration of molecular techniques coupled with clinical insights allows for the development of more effective treatment paradigms tailored to individual patient needs.
Moreover, the exploration of bile acid signaling and its role in liver cancer progression opens the door for innovative diagnostic methods. Understanding the specific mechanisms linking bile acid imbalance and liver pathology could facilitate earlier diagnosis and enable targeted preventative measures. The convergence of basic research and clinical application represents a hopeful pathway toward advancing liver cancer treatment and improving outcomes for patients.
Challenges in Liver Cancer Treatment and Management
The landscape of liver cancer treatment faces several challenges, particularly when addressing the complexities of underlying metabolic disorders. Treatments often focus on the symptoms of liver cancer rather than the metabolic imbalances that can promote tumorigenesis. Given the interplay between bile acid metabolism and liver health, there’s an urgent need for more integrated approaches that tackle the root causes of liver cancer.
Compounding the challenges is the necessity for more research into the long-term impacts of existing treatments on bile acid homeostasis and liver function. As we unearth more about the role of FXR and YAP, understanding how to effectively incorporate these findings into clinical practices will be crucial. Developing comprehensive liver cancer management strategies that consider both metabolic and oncological factors offers the best chance for improving survival rates and patient quality of life.
Frequently Asked Questions
What is the connection between bile imbalances and liver cancer?
Bile imbalances can disrupt bile acid metabolism, leading to liver injury, inflammation, and eventually liver cancer, specifically hepatocellular carcinoma (HCC). Recent studies have highlighted how improper bile acid regulation can trigger these processes.
How do bile acids influence liver cancer treatment?
Bile acids play a critical role in liver cancer treatment by affecting liver metabolism and signaling pathways. For instance, the activation of the Farnesoid X receptor (FXR) can potentially block the harmful effects of bile acid overproduction linked to liver cancer.
What role does the FXR play in bile acid metabolism and liver cancer?
The FXR, or Farnesoid X receptor, is essential for maintaining bile acid homeostasis. When bile acid production is disrupted, FXR function is impaired, contributing to liver fibrosis and escalating the risk of hepatocellular carcinoma (HCC). It’s a key target for new liver cancer treatments.
How does the YAP signaling pathway affect bile imbalances and liver cancer risk?
The YAP signaling pathway regulates liver cell growth and bile acid metabolism. When activated, YAP can inhibit FXR function, leading to bile acid accumulation and increasing the risk of liver cancer. Research suggests that manipulating YAP activity may help in liver cancer prevention.
What strategies are being explored to manage bile imbalances in liver cancer patients?
Strategies include enhancing FXR function, inhibiting YAP’s repressive role, and promoting the export of bile acids through proteins like BSEP. These approaches aim to restore bile acid balance and reduce liver damage, potentially slowing liver cancer progression.
| Key Points | Details |
|---|---|
| Bile Imbalances | Imbalances in bile acids can trigger liver diseases, particularly hepatocellular carcinoma (HCC), the most common form of liver cancer. |
| Study Findings | The research identified a molecular switch that disrupts bile acid regulation, leading to liver injury and cancer. |
| YAP’s Role | YAP, a key protein in the Hippo/YAP pathway, acts as a repressor in bile acid metabolism, inhibiting FXR, which is crucial for bile homeostasis. |
| Implications for Treatment | Potential therapies could involve enhancing FXR function or promoting bile excretion, which might stop liver damage and cancer progression. |
| Research Background | The study was conducted by Yingzi Yang at HSDM, who focuses on the interplay between cell signaling and liver diseases. |
Summary
Bile imbalances and liver cancer are closely intertwined, as recent research has revealed the crucial role bile acids play in liver health. This study highlights an important connection between the disruption of bile acid regulation and the development of hepatocellular carcinoma (HCC). Understanding the mechanisms behind this link, particularly the role of the YAP protein and FXR receptor, opens the door to potential new treatments that could help prevent liver cancer, emphasizing the need for continued exploration in this critical area of health.